Laten we samen een prachtige toekomst creëren!
Inleiding tot leverfunctietests (LFT's)
De lever is een vitaal orgaan dat een centrale rol speelt in het metabolisme, ontgifting en biochemische synthese. Om de optimale prestaties te waarborgen, vertrouwen clinici op leverfunctietests (LFT's) - een groep bloedbepalingen die zijn ontworpen om de leverfunctie te beoordelen, leverbeschadiging te detecteren en ziekteprogressie te bewaken.
Het belang van de leverfunctie bij de menselijke gezondheid
De lever vervoert meer dan 500 fysiologische functies, waaronder de productie van gal, metabolisme van voedingsstoffen en medicijnen, synthese van plasma -eiwitten zoals albumine en regulatie van bloedstolling. Schade of disfunctie in de lever kan deze processen aanzienlijk beïnvloeden, wat leidt tot systemische gezondheidsproblemen. Vroege detectie is de sleutel, waar LFT's in het spel komen.
Rol van leverfunctietests in klinische diagnostiek
LFT's dienen als een primair diagnostisch hulpmiddel in de klinische chemie, waardoor zorgverleners in staat stellen:
Identificeer leverontsteking of schade (bijv. Hepatitis).
Diagnose van chronische leverziekten zoals cirrose of niet-alcoholische leververvetting (NAFLD).
Bewaak de leverfunctie tijdens langdurige behandelingsregimes.
Evalueer de hepatotoxiciteit van farmaceutische producten of blootstelling aan het milieu.
Begeleiding van beslissingen in chirurgische procedures en transplantatie.
Deze tests zijn een integraal onderdeel van in vitro diagnostiek, vaak uitgevoerd met behulp van klinische leverfunctietestkits die een panel van biomarkers in serum of plasma meten.
Soorten leverziekten gedetecteerd door LFT's
LFT -panelen kunnen een breed scala aan leveromstandigheden identificeren, waaronder maar niet beperkt tot:
Hepatitis A, B en C
Alcoholische leverziekte
Cirros
Leverkanker
Cholestase
Auto -immuun hepatitis
Door geneesmiddelen geïnduceerde leverbeschadiging (DILI)
Afhankelijk van de klinische context kunnen artsen een volledig leverpaneel bestellen of specifieke enzymtests zoals ALT, AST of bilirubine om de onderliggende pathologie te bepalen.
Componenten van een leverfunctie -testkit
Leverfunctietestkits zijn gespecialiseerde diagnostische hulpmiddelen die zijn ontworpen om levergerelateerde biomarkers in bloedmonsters kwantitatief te meten. Deze kits worden gebruikt in klinische laboratoria, ziekenhuizen en onderzoeksfaciliteiten om betrouwbare en gestandaardiseerde testen te garanderen.
Elke testkit omvat meestal specifieke reagentia, kalibrators, bedieningselementen en kan speciale apparatuur vereisen, afhankelijk van de testmethode (bijvoorbeeld spectrofotometrie of ELISA). De belangrijkste componenten richten zich op enzymen en eiwitten die wijzen op de gezondheid en functie van de lever.
Kernreagentia opgenomen in een leverfunctie -testkit
Alt -reagens (Alanine Aminotransferase)
Meet het ALT -enzym, dat toeneemt met schade van levercellen.
Gewoonlijk verhoogd bij hepatitis en andere leverwonden.
AST -reagens (aspartaat aminotransferase)
Beoordeelt AST -enzymspiegels, gevonden in lever-, hart- en spierweefsel.
Handig bij het differentiëren van lever versus niet-leiding oorzaken van enzymhoogte.
Bilirubine -reagens
Maatregelen Totaal en directe (geconjugeerde) bilirubine.
Verhoogd in omstandigheden zoals geelzucht, galwegenobstructie en hemolyse.
Albuminereagens
Kwantificeert serumalbumine, een eiwit gesynthetiseerd door de lever.
Lage niveaus kunnen duiden op chronische leverziekte of een slechte synthetische functie.
GGT-reagens (gamma-glutamyltransferase)
Gevoelige indicator van galobstructie en alcoholgerelateerde leverschade.
Vaak gebruikt naast ALP voor een betere diagnostische specificiteit.
ALP -reagens (alkalische fosfatase)
Detecteert ALP -niveaus, geassocieerd met galstroom en botactiviteit.
Hoge ALP suggereert cholestase of infiltratieve leverziekten.
Extra componenten
Kalibrators en normen
Gebruikt om ervoor te zorgen dat de test nauwkeurige en lineaire resultaten oplevert over meetbare bereiken.
Controlemateriaal
Kwaliteitscontrolemonsters met bekende concentraties om de consistentie en betrouwbaarheid van de test te verifiëren.
Buffers en verdunningsmiddelen
Handhaaf de optimale pH- en reactieomstandigheden voor elk specifiek enzym.
Stabilisatoren en conserveermiddelen
Voorkom degradatie van reagens en handhaven de houdbaarheid onder opslagomstandigheden.
Vereiste apparatuur en verbruiksartikelen
Om assays betrouwbaar uit te voeren, vereisen laboratoria meestal:
Spectrofotometer (voor op absorptie gebaseerde detectie)
Microplaatlezer (voor ELISA -kits)
Geautomatiseerde chemieanalysatoren
Cuvettes, pipetten en tips
Centrifuge (voor serum/plasma -scheiding)
Incubator (voor temperatuurgestuurde reacties)
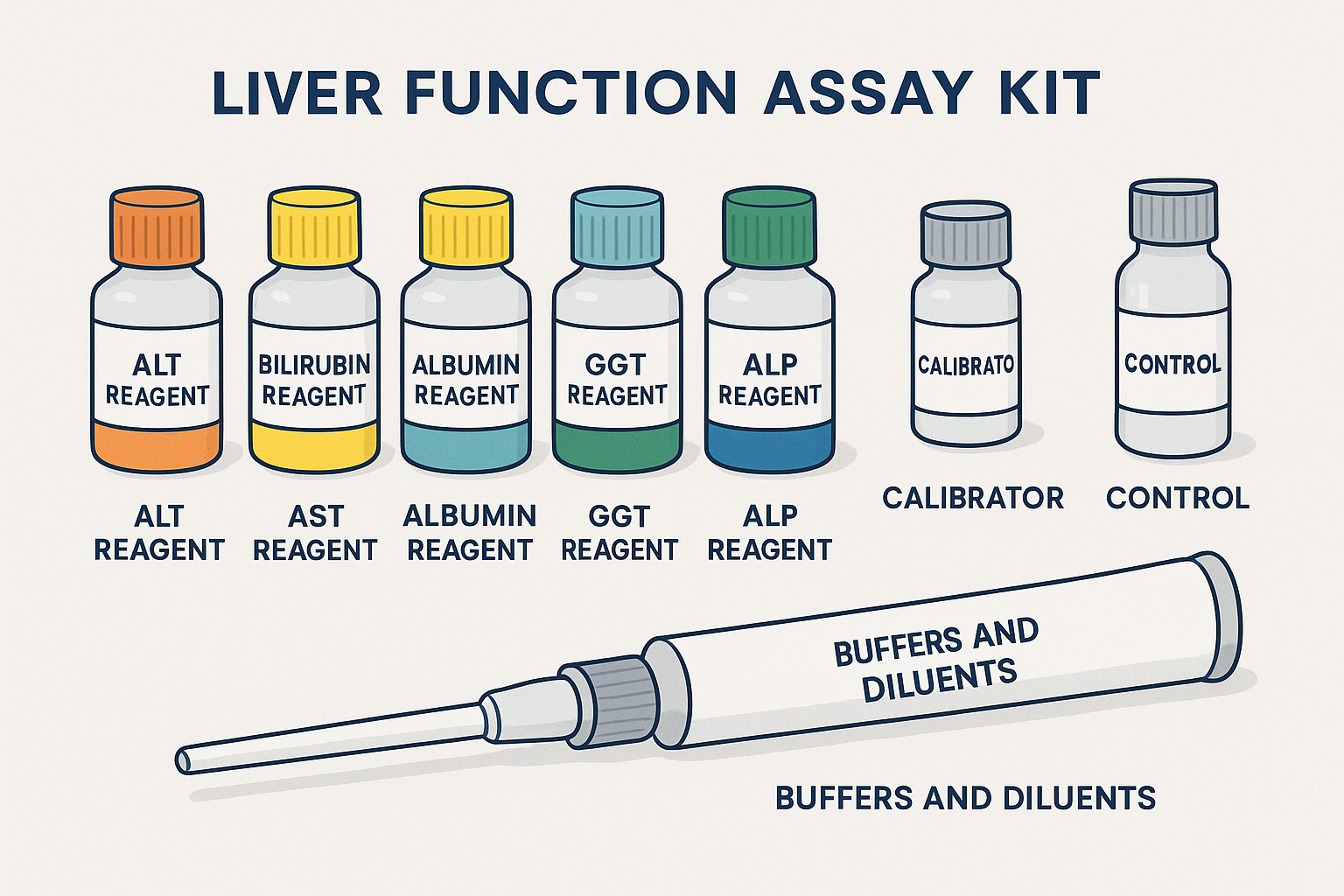
Soorten leverfunctietestkits
Leverfunctie assay kits Kom in verschillende formaten, elk op maat gemaakt om aan specifieke klinische behoeften te voldoen-van ziekenhuislaboratoria met hoge doorvoer tot gedecentraliseerde point-of-care-instellingen. Inzicht in de verschillende typen helpt clinici het juiste platform te kiezen op basis van nauwkeurigheid, snelheid, schaalbaarheid en kosteneffectiviteit.
Spectrofotometrische testkits
Dit zijn het meest voorkomende type leverfunctietestkits dat in klinische laboratoria wordt gebruikt.
Principe: meet de absorptie van een gekleurd reactieproduct met behulp van een spectrofotometer.
Toepassingen: kwantificering van enzymen zoals ALT, AST, ALP en GGT.
Voordelen:
Hoge gevoeligheid en reproduceerbaarheid.
Geschikt voor automatisering bij klinische chemieanalysatoren.
Beperkingen:
Vereist gekalibreerde spectrofotometrische apparatuur.
Handmatige monsterafhandeling kan de variabiliteit vergroten indien niet geautomatiseerd.
Op ELISA gebaseerde assaykits (enzym-gekoppelde immunosorbens-test)
Ontworpen voor het meten van levergerelateerde antigenen of eiwitten (bijv. Hepatitis markers, albumine).
Principe: antilichaam-antigeen interacties met enzymatische signaalversterking.
Toepassingen: detectie van levereiwitten en inflammatoire markers.
Voordelen:
Hoge specificiteit.
Kan zeer lage concentraties detecteren (picogramspiegels).
Beperkingen:
Complexer protocol.
Langere doorlooptijd.
Geautomatiseerde klinische chemieanalysekits
Compatibel met laboratoriumsystemen met high-throughput zoals Roche Cobas, Beckman AU of Siemens Advia.
Principe: integratie van spectrofotometrische methoden met geautomatiseerde vloeistofafhandeling.
Toepassingen: routinematige testen in ziekenhuizen en diagnostische centra.
Voordelen:
Snel, geautomatiseerd en schaalbaar.
Minimale hands-on tijd.
Beperkingen:
Vereist dure apparatuur.
Minder flexibiliteit bij het aanpassen van assays.
Point-of-Care Testing (POCT) kits
Portable, snelle-tests die zijn ontworpen voor bijna-patiënt testen.
Principe: kan droge chemie, laterale stroom of geminiaturiseerde optische sensoren gebruiken.
Toepassingen: spoedkamers, poliklinieken en woningmonitoring.
Voordelen:
Snelle resultaten (vaak binnen enkele minuten).
Eenvoudig te gebruiken zonder gespecialiseerde apparatuur.
Beperkingen:
Beperkt paneel (vaak alleen alt of bilirubine).
Lagere precisie vergeleken met lab-gebaseerde testen.
Procedure voor het uitvoeren van een leverfunctietest
Het uitvoeren van een leverfunctietest vereist zorgvuldige monsterafhandeling, precieze reagensvoorbereiding en gestandaardiseerde analytische procedures om nauwkeurige en reproduceerbare resultaten te garanderen. Hieronder is een algemene handleiding die van toepassing is op de meeste leverfunctie -testkits, met name die gebaseerd op spectrofotometrische of geautomatiseerde chemieanalyseplatforms.
Voorbeeldverzameling en voorbereiding
Voorbeeldtype:
Preferred: Serum
Alternatief: plasma (gehypepariniseerd of met EDTA behandeld)
Verzamelingsinstructies:
Verzamel 3-5 ml veneus bloed met behulp van steriele vacutainer -buizen.
Laat het bloed stolven bij kamertemperatuur (voor serum).
Centrifuge bij 3000 tpm gedurende 10 minuten om serum of plasma te scheiden.
Opslag en behandeling:
Analyseer monsters binnen 1-2 uur voor de beste resultaten.
Als de vertraging wordt verwacht, koel je bij 2-8 ° C (stabiel gedurende 24-48 uur).
Vermijd hemolyse of lipemia, die kunnen interfereren met optische metingen.
Stapsgewijze handleiding voor het uitvoeren van de test
Reagensvoorbereiding
Breng reagentia voor gebruik naar kamertemperatuur (18-25 ° C).
Meng zachtjes zonder schuimen.
Volg kitspecifieke instructies voor reconstitutie als reagentia worden gelyofiliseerd.
Kalibratie
Gebruik de meegeleverde kalibrators of een standaardcurve om de absorptie van basislijn te definiëren.
Voer blanco, standaard en monstermetingen uit.
Kalibratie moet dagelijks worden geverifieerd of na veranderingen in de reagens.
Steekproefmeting
Pipet gespecificeerde volumes van reagens en monster in cuvettes of microplaatputten.
Incubeer het reactiemengsel bij gedefinieerde temperatuur (vaak 37 ° C) voor de vereiste tijd.
Meet de absorptie bij de juiste golflengte met behulp van een spectrofotometer of geautomatiseerde analysator.
Voorbeeld golflengten:
ALT/AST: 340 nm
Bilirubin: 540 nm
Albumine: 630 nm
ALP/GGT: 405 nm
Bereken de enzymactiviteit of analytconcentratie met behulp van de kalibratiecurve of absorptieverschilmethode.
Kwaliteitscontrole en validatie
Gebruik van controlemateriaal:
Voer lage en hoog niveau bedieningselementen uit bij elke partij tests.
Zorg ervoor dat de resultaten binnen acceptabele reeksen vallen die door de fabrikant zijn gedefinieerd.
Herhaalbaarheid:
Duplicaat testen kunnen helpen bij het identificeren van pipetting- of reagensfouten.
Instrumentonderhoud:
Regelmatige reiniging en kalibratie van spectrofotometers of analysatoren is cruciaal.
Documentatie:
Noteer partijnummers, besturingswaarden en testomstandigheden voor traceerbaarheid en auditdoeleinden.
Interpretatie van resultaten
Nauwkeurige interpretatie van de resultaten van de leverfunctietest (LFT) is essentieel voor het diagnosticeren van leveraandoeningen, het monitoren van ziekteprogressie en het evalueren van behandelingsresultaten. Elke parameter in een leverpaneel biedt inzicht in verschillende aspecten van de leverfunctie en schade. Hieronder vindt u een uitsplitsing van normale bereiken, klinische implicaties en beïnvloedende factoren voor elke belangrijke marker.
Normale reeksen voor leverfunctieparameters
| Parameter | Normaal bereik (volwassenen) | Klinische betekenis |
| Alt (Alanine Aminotransferase) | 7–56 u/l | Verhoogd in hepatocellulair letsel (bijv. Virale hepatitis, door drugs veroorzaakte leverschade). |
| Ast (aspartaat aminotransferase) | 10–40 u/l | Neemt toe met lever-, hart- of spierschade; High AST: ALT -verhouding kan suggereren dat alcoholische leverziekte. |
| Bilirubine (totaal) | 0,3-1,2 mg/dl | Verhoogd in geelzucht, galwegenobstructie of hemolyse. |
| Albumin | 3.5–5.0 g/dl | Verlaagd bij chronische leverziekte als gevolg van een verminderde synthetische functie. |
| GGT (gamma-glutamyltransferase) | 9–48 u/l | Gevoelig voor alcoholgebruik, galobstructie en enzyminductie door medicijnen. |
| ALP (alkalische fosfatase) | 44–147 U/L | Verhoogd in cholestase en botaandoeningen; vaak geïnterpreteerd naast GGT. |
Opmerking: referentiebereiken kunnen variëren per leeftijd, geslacht, bevolking en laboratoriumkalibratie.
Factoren die de LFT -resultaten beïnvloeden
Fysiologische variabelen:
Leeftijd, geslacht en zwangerschap kunnen de enzymspiegels beïnvloeden.
Voorbeeldintegriteit:
Hemolyse kan AST en Alt valselijk verhogen.
Lipemische of icterische monsters kunnen spectrofotometrische metingen verstoren.
Medicijnen en alcohol:
Statines, antibiotica, anti-epileptica en alcohol kunnen leverenzymen verhogen.
Vasten en houding:
Niet-fasterende monsters kunnen albumine- en bilirubine-metingen enigszins beïnvloeden.
Klinische betekenis van abnormale resultaten
Hepatocellulair letsel (bijv. Virale hepatitis, Dili):
Gemarkeerde toename van ALT en AST, vaak met alt> ast.
Cholestasis of galwegen obstructie:
Verhoogde ALP en GGT, met mild verhoogde bilirubine.
Alcoholische leverziekte:
AST: ALT -verhouding> 2: 1, verhoogde GGT.
Cirrose en leverfalen:
Verlaagd albumine, langdurige PT/INR (niet in standaard LFT-kits), milde tot matige verhogingen in enzymen.
Hemolyse of hematologische aandoeningen:
Verhoogde indirecte bilirubine zonder verhoogde ALT/AST.
Toepassingen van leverfunctietestkits
Leverfunctietestkits spelen een cruciale rol in zowel klinische als onderzoeksinstellingen. Door snelle en betrouwbare inzichten in de leverfunctie te bieden, ondersteunen deze kits een breed scala aan diagnostische en monitoringtoepassingen tussen patiëntenpopulaties.
Diagnose van leverziekten
Leverfunctietests zijn essentiële hulpmiddelen bij het identificeren van verschillende leverziekten, waardoor vroege interventie en behandeling mogelijk is.
Virale hepatitis (A, B, C):
Verhoogde ALT en AST, vaak met milde bilirubine -verhoging.
Gebruikt om leverontsteking te bevestigen en herstel te bewaken.
Cirrose:
Verminderd albumine en verhoogde bilirubine, soms met mild verhoogde enzymen.
Helpt bij het beoordelen van de progressie van chronische leverschade.
Cholestase en galwegen obstructie:
Hoge ALP en GGT, met mogelijke toename van bilirubine.
Suggereert een verminderde galstroom en vereist beeldvormingsbevestiging.
Niet-alcoholische leververvetting (NAFLD):
Milde tot matige ALT/AST -hoogte.
Vaak gediagnosticeerd naast beeldvorming en metabole tests.
Monitoring van de levergezondheid in chronische aandoeningen
Voor patiënten met een bekende leverziekte of patiënten die langdurig worden behandeld, zijn LFT's cruciaal bij het volgen van de leverstatus.
Auto -immuun hepatitis:
Regelmatig volgen van leverenzymen helpt de immuunactiviteit en de effectiviteit van de behandeling te beoordelen.
Levertransplantatiepatiënten:
Frequente LFT's detecteren vroege tekenen van afwijzing of transplantaatdisfunctie.
Chronische hepatitis B/C:
Lopende monitoring zorgt voor effectieve virale onderdrukking en voorkomt complicaties.
Beoordeling van door geneesmiddelen geïnduceerde leverbeschadiging (DILI)
Veel geneesmiddelen dragen het risico op hepatotoxiciteit. LFT's zijn verplicht voor:
Baseline beoordeling voordat hepatotoxische geneesmiddelen worden gestart (bijv. Methotrexaat, Isoniazid).
Routinematige monitoring tijdens de behandeling voor vroege detectie van bijwerkingen.
Veiligheidsevaluaties voor geneesmiddelen in klinisch onderzoek.
Evaluatie van alcoholgerelateerde leverziekte
GGT, AST en de AST: ALT-verhouding worden vaak gebruikt om de mate van door alcohol geïnduceerde leverschade te evalueren. Verhoogde GGT -niveaus dienen ook als een biomarker voor chronisch alcoholgebruik, zelfs voordat structurele schade duidelijk is.
Onderzoekstoepassingen in hepatologie
In academisch en farmaceutisch onderzoek worden LFT -assaykits gebruikt om:
Karakteriseren ziektemodellen in preklinische studies.
Scherm hepatoprotectieve middelen bij het ontdekken van geneesmiddelen.
Meet biomarkers voor klinische onderzoeken met levermetabolisme of toxiciteit.
 |  |
Voordelen en beperkingen van leverfunctietestkits
Leverfunctie -assaykits bieden gestroomlijnde en gestandaardiseerde oplossingen voor het beoordelen van de gezondheid van de lever, maar zoals elk diagnostisch hulpmiddel, hebben ze sterke punten en potentiële nadelen. Inzicht in deze kan clinici, laboratoria en onderzoekers begeleiden bij het selecteren en interpreteren van de juiste test voor de juiste context.
Voordelen van het gebruik van leverfunctietestkits
Standaardisatie en betrouwbaarheid
Kits zijn vooraf gealalideerd met gedefinieerde protocollen en reagentia.
Zorg voor consistente prestaties tussen laboratoria en technici.
Hoge gevoeligheid en specificiteit
De meeste kits zijn ontworpen om minuutconcentraties van leverenzymen of eiwitten te detecteren.
Vooral waar voor op ELISA gebaseerde of geautomatiseerde chemie-kits.
Compatibiliteit met automatisering
Ideaal voor laboratoria met hoge doorvoer met behulp van klinische chemieanalysatoren.
Verminder de handmatige fout en verbetert de doorlooptijd.
Uitgebreide panelen
Multi-analytkits maken gelijktijdig testen van ALT, AST, ALP, GGT, Bilirubin en albumine mogelijk.
Bespaar tijd en monstervolume.
Point-of-Care Testing (POCT) -opties
Snelle tests voor GGT, ALT of bilirubine zijn beschikbaar voor klinieken en noodgevallen.
Schakel snellere klinische beslissingen in.
Goedkeuring
Veel kits zijn CE-gemarkeerd, door de FDA goedgekeurd of IVD-gecertificeerde, waardoor veiligheid en klinische effectiviteit worden gewaarborgd.
Beperkingen en mogelijke foutenbronnen
Pre-analytische variabiliteit
Kwaliteitskwaliteitsproblemen zoals hemolyse, lipemia of langdurige opslag kunnen de resultaten scheeftrekken.
Onjuiste monsterafhandeling is een veel voorkomende bron van valse positieven of negatieven.
Biologische variabiliteit
Enzymniveaus kunnen fluctueren op basis van geslacht, leeftijd, dieet, lichaamsbeweging en comorbiditeiten.
Vereist voorzichtige interpretatie zonder overredheid van afzonderlijke waarden.
Interferentie en kruisreactiviteit
Medicijnen, alcohol of auto -antilichamen kunnen interfereren met colorimetrische of immunoassays.
Kan leiden tot vals verhoogde of onderdrukte waarden.
Beperkte functionele beoordeling
Standaard LFT -kits meten biochemische markers, maar beoordeel niet direct synthetische of uitscheidingsleverfuncties zoals stollingsfactoren of ammoniakklaring.
Instrumentafhankelijkheid
Spectrofotometrische en geautomatiseerde kits vereisen goed onderhouden, gekalibreerde apparatuur.
De prestaties kunnen afbreken als systemen niet regelmatig worden gecontroleerd of gevalideerd.
Vergelijking met andere diagnostische methoden
| Methode | Voordelen | Beperkingen |
| LFT -testkits | Snel, kwantitatief, gestandaardiseerd | Beperkt tot enzym/eiwitniveaus |
| Imaging (echografie, MRI) | Structurele visualisatie | Kan geen vroege biochemische veranderingen detecteren |
| Leverbiopsie | Histologisch detail | Invasieve, bemonsteringsfout |
| Fibroscan / elastografie | Niet-invasieve fibrose-detectie | Beperkte beschikbaarheid, geen directe functionele test |



